Lecture Details[]
Ramesh Rajan; Week 2 MED1022; Physiology
Lecture Content[]
Pain is subjective and protective in nature. Peripheral pain input goes through SC processing, perception is affected by the cognitive system, motivational-affective system and sensory-discriminative system, output to motor response mechanism.
Pattern theory is pain is experienced when stimulus is intense enough, specificity theory says there is a specific receptor for different pain stimuli, gate control theory says substantia gelatinosa in spinal cord is a gate which can open to transmit pain, affect theory is that pain is emotional and pain intensity depends on the value of the organ, parallel processing suggests physiologic and neurologic pain deciphering occurs separately.
Phases of nociception are transduction phase (noxious stimuli excite nociceptors), transmission phase (pain to SC, SC to brainstem and thalamus, thalamus to sensory cortex). Modulation phase (descending system which amplifies or inhibits pain) and perception phase (patient becomes conscious to pain).
Pain receptors are generally free nerve endings. Unencapsulated nerve endings are most abundant of all somatic receptors, are polymodal (respond to many stimuli). Nociceptors only respond to stimuli at damaging levels. Noxious stimuli are transduced into electrical activity at C and Adelta receptors. Different ion channels transduce the effects of different noxious agents. Entry of Na excites the receptors. Noxious stimuli also triggers release of inflammatory mediators from neighbouring tissue, including serotonin, K, PG and cytokines which cause movement of ions across the cell membrane. Inflammatory mediators can change transduction of pain. Some anti-nociceptive agents (NSAIDs + COXII inhibitors) block these mediators.
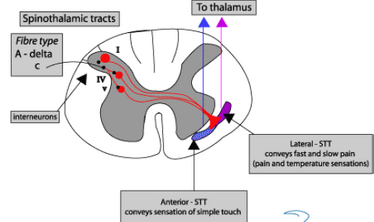
A delta axons are high threshold mechanoreceptors, thermal and thermal-mechanical, C are polymodal nociceptors. C fibres terminate in SC at II laminae, delta at I and V. The second order neurons they connect with also recieve signals from Aa and Ab fibres. They modulate the pain signal. Glutamate is the main transmitter at the dorsal horn, acts on AMPA receptors to allow Na influx, depolarising the projection neuron. With sustained signalling, Glu also activates NMDA receptors (Ca influx), leading to LTP of pain transmission. Substance P is also present, released from C terminals after stronger/more sustained stimuli activates NK-1 receptor. It enhances Glu transmission across synapse.
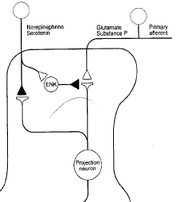
A beta can synapse on inhibitory interneurons to pain which connect to the STT where pain signals converge. There are two main ascending pain pathways- spinothalamic, which transmits to cortical pathways associated with discrimination and affect; spinoparabrachial pathways from superficial dorsal horn which is associated with affect. There are many cortical connections with the pathways: insula for perception, primary somatosensory cortex for localisation, singulate frontal cortex for affective and emotional elements of pain. Modulation phase is in the descending pathway, excitatory glial cell amino acids persist or amplify pain. The main descending system is from amygdala/hypothalamus, terminates in periaqueductal grey (PAG) which
project to the lower brainstem to control anti-nociceptive and autonomic responses that follow noxious stimulation. Brainstem adrenergic and serotonergic neurons excite enkephalinergic neurons in the SC and inhibit STT neurons. ENK prevents transmission from pain fibres to STT neurons, so STT neurons are double inhibited from the brainstem.
Opiates reduce duration of sensory neuron actions potential. They also hyperpolarise dorsal horn neurons and decrease afferent evoked EPSP.
A delta fibres are high threshold mechanoreceptors, thermal, thermal-mechanical. They have a high threshold and activate AMPA and NMDA and mGlu. They transmit fast pain. C fibres are polymodal nociceptors, activate NK1 and 2 and convey slow pain. Abeta has a low threshold, AMPA, touch, vibration and pressure.
Referred pain is probably due to the visceral structure and the surface structure sending pain input to the same dorsal horn neuron. Nociceptors can be sensitised by prior injury , can be from local inflammation and sensitisation of nociceptors at damage site. This sensitisation is primary hyperalgesia. The surrounding area can also become sensitised (secondary hyperalgesia) due to changes in CNS processing of information from the surrounding sites. Sensitisation leads to increased spontaneous impulse activity, enhanced responses to impulses in primary afferents; causes hyperalgesia, allodynia and spontaneous pain. Sensitisation is due to buildup of intracellular Ca due to ongoing C nociceptive input, repeated NMDA activation, protein kinase C sensitisation of NMDA, STT neuron becomes hyperresponsive to primary nociceptive neurons and peripheral painful stimuli. LTP can be prevented if release of glutamate/substance P is inhibited eg. by activation of G protein coupled mu opioid receptors, or if opening of Ca permeable ion channels is blocked eg by post synaptic opioid inhibition